E-Newsletter - July 2019
ADDING Value to the Alliance Clinical Trial Process
ALLIANCE PATIENT ADVOCATE COMMITTEE
Patient advocacy is an integral part of the Alliance for Clinical Trials in Oncology. Alliance Patient Advocates are cancer survivors and caregivers and come from all walks of life. They play an active role in cancer research by bringing the patient's perspective to the investigative and clinical environment. Patient advocates help clinical researchers find better answers for people with cancer more quickly.
Who We Are

Alliance Patient Advocates:
• Attend and participate in scientific meetings
• Review study concepts and protocols
• Assist in designing study accrual strategies
• Develop plain language study results summaries
• Make presentations to interested groups of scientists and clinicians
Our Three Defining Aims:
1. Promote patient advocate participation as full members of Alliance disease and modality/discipline committees;
2. Communicate individually with constituent patient populations to maintain currency of input and validity of voice for the collective patient experience; and
3. Allow Alliance patient advocates to remain current on the science of cancer research.
How We Participate within the Alliance
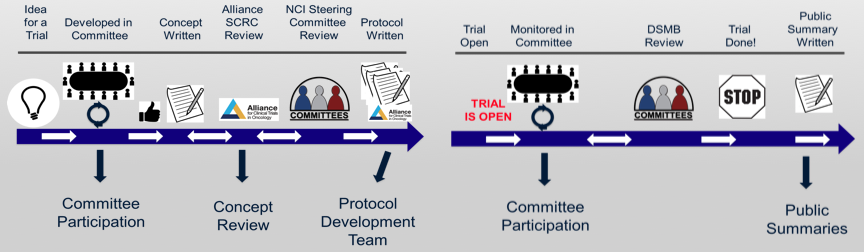
Participating in Committee Meetings
• Attend all primary committee meetings, in-person and by teleconference.
• Actively participate in discussions.
• Provide the patient perspective.
• Bring a broad community perspective to the conversation.
Concept Review. Alliance patient advocates serve as evaluators of phase II or III clinical trial concepts. Below are the suggested topics reviewed by patient advocates. Points of emphasis will vary from concept to concept. However, the following five main headings are criteria used to guide the advocate review.
• Study Impact
• Study Feasibility
• Level of Innovation
• NCTN Relevance
• Study Design
Protocol Review Committee
• Review the protocol as it is written.
• Look at eligibility criteria.
• Review questionnaires.
• Review schedule for patient burden.
• Review the consent and re-write parts when necessary to ensure a good readability level.
Patient Perspective
• Think about the outcome and whether it will be important to patients and make a difference.
• Will participation be hard for patients? (lots of visits, mandatory biopsies, etc.)
• Is there randomization? If so, will it be acceptable to patients?
• Is the trial complicated? Is there a need to help explain to patients?
• Will there be high “costs” to patients? Are scans covered?
• What is the patient population? Who is excluded/included? Is it appropriate? Will special outreach be needed?
• What are the eligibility criteria? If it seems unnecessary, ask why it’s there. (brain mets exclusion, prior therapy, line of therapy, age, sex)
Public Summaries
• Publications Committee (via Deb Collyar)
• Template (via Deb Collyar)
• Written in plain language
• Written after or at the same time as the publication
• Written by a “team” including a patient advocate
• Posted on the Alliance website
For other articles in this issue of the Alliance E-News newsletter, see below.
- Message From the Group Chair - Monica M. Bertagnolli, MD
-
PI Perspective: Alliance Health Outcomes Committee
Michelle Naughton, PhD and Amylou Dueck, PhD (Co-Chairs)
Jackie Lafky, MS (Program Manager) -
Changing the Guard: Director and PI, Alliance Central Protocol Operations Program
Gin F. Fleming, MD and Olwen Hahn, MD - Recent Alliance Protocol Activations
-
Spotlight on Alliance Trials
Alliance A091802 - Cutaneous squamous cell carcinoma
Alliance A041701 - Chronic myeloid leukemia
Alliance A031704 - Advanced renal cell cancer
Alliance A031702 - Rare genitourinary tumors - ICAREdata | Integrating Clinical Trials and Real World Endpoints
-
Adding Value to the Alliance Clinical Trial Process
Alliance Patient Advocate Committee -
Announcements
-- Alliance Joins with ASCO, CancerLinQ and The MITRE Corporation to Publish Initial Set of Common Cancer Data Standards and Specifications Comprising mCODE (VIDEO))
-- Recap: Alliance and Alliance Foundation Trials at 2019 ASCO
-- Renewed NCI Investigational Agent Accountability Record Forms (DARFs)
-- Call for Nominations + Applications: 2019/2020 Alliance Awards + Honors
-- Alliance Appoints New Committee Chairs -
Alliance in the News
-- GNS Healthcare Chosen to Present Discovery of New Clinical Predictors of Overall Survival in Metastatic Colorectal Cancer at ESMO 2019 in Collaboration with the Alliance for Clinical Trials in Oncology
-- mCODE Improves EHR Data Standardization for Cancer Research
-- First Patient Dosed in Phase III Study of E-Selectin Antagonist Uproleselan in AML (Alliance A041701)--ASCO 2019: CALGB 90601 (Alliance): Randomized, Double-blind, Placebo-controlled Phase III Trial Comparing Gemcitabine and Cisplatin with Bevacizumab or Placebo in Patients with Metastatic Urothelial Carcinoma
--'Magic Mouthwash' Little Help for Radiation-Induced Mucositis (Alliance A221304)
-- Magic mouthwash effective treatment for mouth sore pain caused by radiation therapy (Alliance A221304)
-- Mouthwashes Show Promise in Reducing Chemo-Related Oral Mucositis Pain (Alliance A221304)
-- Giving Drugs before Surgery Lowers the Risk of Prostate Cancer Returning (CALGB 90203)
-- Study: Identifying colorectal cancer subtypes could improve treatment (CALGB/SWOG 80405)
-- The Fight to Save the Bladder Turns to Genomics (Alliance A031701)


