E-Newsletter - Summer 2017
What Alliance Members Need to Know
About CTEP's New Registration and Credential Repository (RCR)
The CTEP Registration and Credential Repository (RCR) is an on-line registration system designed to improve regulatory compliance during the conduct of NCI-supported studies.
The goals of the systems are to:
- Provide online person registration capabilities
- Provide multi-level quality control checks and electronic validation for investigator qualifications
- Improve functionality of current registration system to allow non-physicians to act as the site-study Principal Investigator (PI) or enrolling PIs for appropriate studies
- Improve documentation of Human Subject Protection (HSP) and Good Clinical Practice (GCP) training requirements
- Improve compliance with federal regulations
RCR will have five (5) registration types:
- Investigator (IVR)
- Non-physician Investigator (NPIVR)
- Associate Plus (AP)
- Associate (A)
- Associate Basic (AB)
The table below provides a description of the registration types and the required regulatory submissions for each
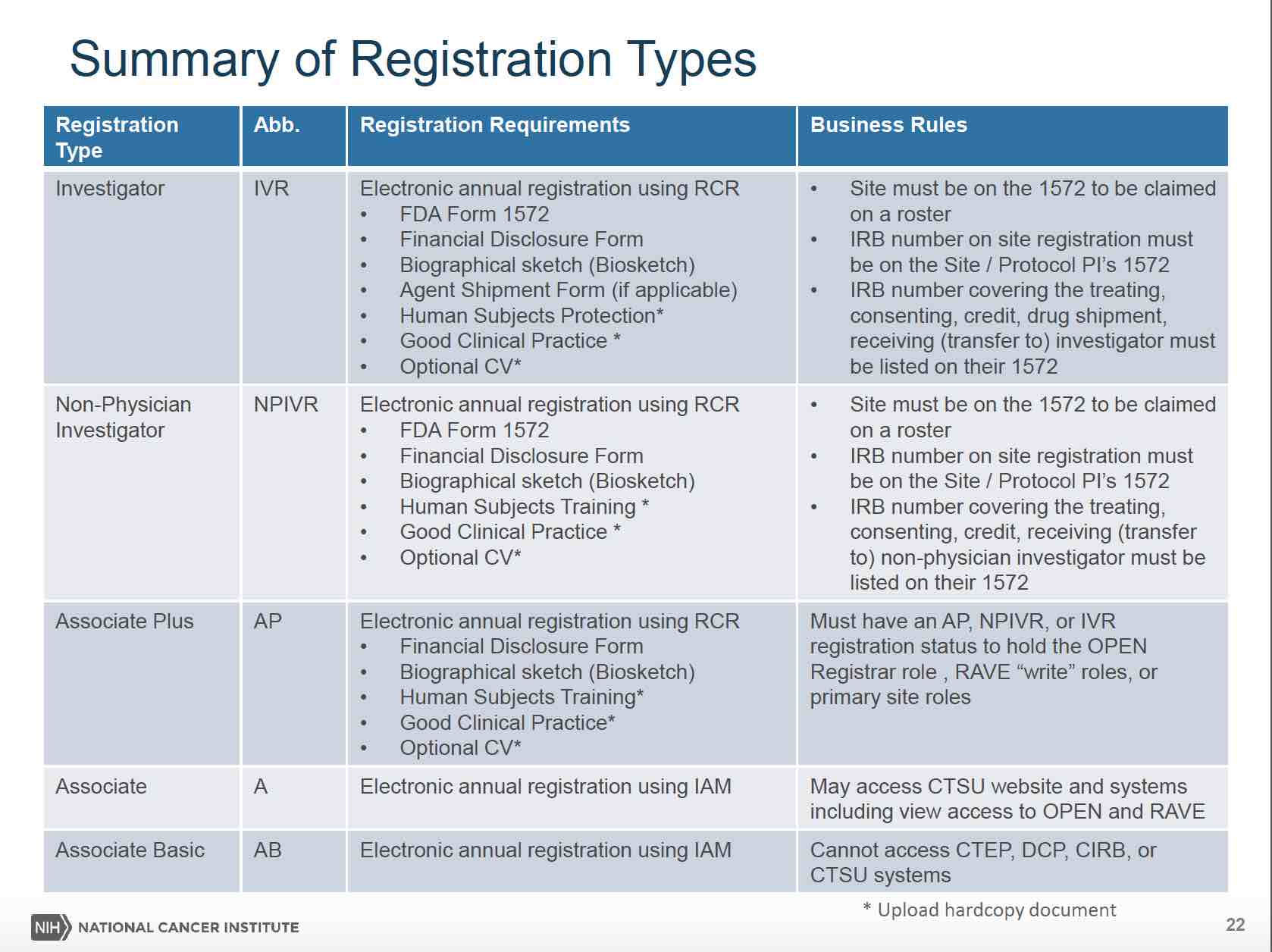
The RCR replaces the manual submission of investigator regulatory submissions of 1572 forms, Financial Disclosure, CV/Biosketch, etc. These documents will be completed online using an electronic signature based on each person’s CTEP IAM sign on. In addition, the Human Subject Protection Training certificate and Good Clinical Practice Training certificate will need to be uploaded to RCR.
The RCR launched on Monday, July 31, 2017.
All individuals involved in the conduct of NCI-supported trials as an Investigator (IVR), Non-Physician Investigator (NPIVR), or Associate Plus (AP) will utilize the RCR application to re-register at the time of their next annual registration.
As a reminder - to better prepare staff at your organization to use RCR, CTEP recommends the following steps:
- Collect Human Subjects’ Protection (HSP) and Good Clinical Practice (GCP) training certificates for all IVRs, NPIVRs, and APs;
- Ensure that all IVRs have CTEP IAM accounts. (*Hint – if your IVR is not sure if they have an IAM account, have them access the IAM application at https://eapps-ctep.nci.nih.gov/iam/, select “Request New Account”, and follow the steps to begin an account request (they will need their CTEP investigator ID). IAM will indicate if an account is already setup for the IVR.);
- Create a cheat sheet of practice sites with their CTEP site code and site name (use RUMS to prepare the site list), institutional laboratories with their CLIA numbers (contact your local labs if needed), and Institutional Review Boards with their IRB numbers (contact your local IRBs if needed);
- Setup a Registration Coordinator (RC) for your investigators by e-mailing CTEPRegHelp@ctep.nci.nih.gov with a subject line of “Make Me a Registration Coordinator” and include the RCs full name, CTEP person ID, CTEP site code, and a list of investigators (CTEP investigator ID and full name) for whom they will be the RC;
- Setup a Primary Shipping Designee for your clinical sites(s) by e-mailing CTEPRegHelp@ctep.nci.nih.gov with a subject line of “Establishing a Primary Shipping Designee for CTEP {Site Code} and CTEP {Site Name}” and include the Shipping Designee’s full name and CTEP person ID (*Hint – a pharmacist with a pharmacy shipping address is strongly preferred.).
Information on common HSP and GCP training modules and additional information on RCR may be found on the Alliance website (member side) at: https://www.allianceforclinicaltrialsinoncology.org/main/member/standard.xhtml?path=%2FMember%2FIndividual-Participation
For other articles in the Summer issue of the Alliance E-News newsletter, see below.
-
Spotlight on Alliance Trials
CALGB (Alliance) 10603 | FDA Approves New Combination Treatment for AML
Alliance 221208 | Managing Brain Radionecrosis (BeSt Trial)
Alliance A091202 | Intervention for Myxoid Liposarcoma
Alliance A091305 | Rare Tumor Subset for Advanced Anaplastic Thyroid Cancer - In the News
-
Protocols
Recent Alliance Protocol Activations - Alliance at ASCO 2017
-
Staff & Members
Meet New Alliance Board of Directors and Executive Committee
Meet New Alliance Leadership
Alliance Members on the Move -
Announcements
What Alliance Members Need to Know About CTEP's New Registration and Credentialing Repository (RCR) -
Featured Columns
Alliance Breast Committee Member Survey
Alliance Disparities Committee Corner - Did You Know? Alliance Website Tips
- Alliance Mission: By the Words


