E-Newsletter - Summer 2017
BeSt (Alliance A221208) - Randomized Phase II Study: Corticosteroids + Bevacizumab vs. Corticosteroids + Placebo (BeSt) for Radionecrosis After Radiosurgery for Brain Metastases
NOW ENROLLING!
Usually the first treatment for brain radionecrosis only works for some patients and can cause significant side effects in many patients.
What is brain radionecrosis?
Brain radionecrosis is a non-cancerous condition in which an area of dead tissue in the brain, caused by treatment with radiation therapy, is surrounded by inflamed (swollen) tissue. Brain radionecrosis can cause headaches, nausea, vomiting, weakness and other neurological symptoms. Corticosteroids, drugs given to reduce inflammation, or swelling, are the most common treatment for brain radionecrosis.
What is the BeSt trial?
The BeSt Trial (Alliance A221208) will study how well corticosteroids and a drug called bevacizumab work compared to corticosteroids alone in improving symptoms in patients with radionecrosis after radiation surgery for cancer that has spread from the original tumor to the brain.
Bevacizumab may help reduce inflammation and lessen the symptoms of radionecrosis by reducing leaking of the blood vessels. It is not yet known whether giving corticosteroids alone or with bevacizumab is more effective in treating radionecrosis in this patient population.
In the BeSt trial, the effects of bevacizumab with standard corticosteroid therapy will be compared to a placebo with standard corticosteroid therapy.
What is the main trial objective?
The primary objective of the BeSt trial (Alliance A221208) is to determine whether adding bevacizumab to standard corticosteroid therapy results in improved symptoms compared with standard corticosteroid therapy.
How will patients be treated on the BeSt trial?
Patients will be randomized (flip of a coin) to one or two treatment groups.
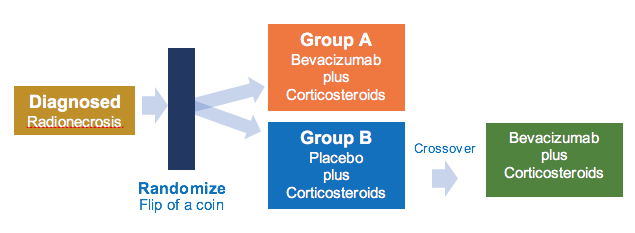
Group A: Patients receive bevacizumab intravenously (IV) over 30-90 minutes on days 1 and 15. Patients also receive dexamethasone or prednisone (corticosteroids) orally (PO) once daily (QD) or twice daily (BID) on days 1-28. Treatment repeats every 28 days for 4 courses in the absence of disease progression or unacceptable toxicity.
Group B: Patients receive placebo IV over 30-90 minutes on days 1 and 15. Patients also receive dexamethasone or prednisone (corticosteroids) PO QD or BID on days 1-28. Treatment repeats every 28 days for four (4) courses in the absence of disease progression or unacceptable toxicity. Patients in Arm II with clinical progression may cross-over to Arm I.
After completion of study treatment, all patients are followed up every two (2) months for six (6) months and then up to one (1) year.
To learn more about the trial and trial locations, visit:
ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02490878
_________________
Image courtesy of www.clipartix.com
For other articles in the Summer issue of the Alliance E-News newsletter, see below.
-
Spotlight on Alliance Trials
CALGB (Alliance) 10603 | FDA Approves New Combination Treatment for AML
Alliance 221208 | Managing Brain Radionecrosis (BeSt Trial)
Alliance A091202 | Intervention for Myxoid Liposarcoma
Alliance A091305 | Rare Tumor Subset for Advanced Anaplastic Thyroid Cancer - In the News
-
Protocols
Recent Alliance Protocol Activations - Alliance at ASCO 2017
-
Staff & Members
Meet New Alliance Board of Directors and Executive Committee
Meet New Alliance Leadership
Alliance Members on the Move -
Announcements
What Alliance Members Need to Know About CTEP's New Registration and Credentialing Repository (RCR) -
Featured Columns
Alliance Breast Committee Member Survey
Alliance Disparities Committee Corner - Did You Know? Alliance Website Tips
- Alliance Mission: By the Words


