E-Newsletter - Summer 2017
Alliance Breast Committee Member Survey:
What Studies Do We Need to Do?
Ann H. Partridge, MD, MPH (Dana-Farber Cancer Institute) and Lisa A. Carey, MD (Lineberger Cancer Center, University of North Carolina) | Co-Chairs, Alliance Breast Committee

 This work was published in part in Clinical Cancer Research in June 2017.
This work was published in part in Clinical Cancer Research in June 2017.
"Unmet Needs in Clinical Research in Breast Cancer: Where Do We Need to Go?"
Partridge, AH, Carey LA
Clin Cancer Res. 2017 Jun 1;23(11):2611-2616. doi: 10.1158/1078-0432.CCR-16-2633.
Clinical trials in the cooperative group setting are dominated by novel drug or regimen trials focused on improving efficacy, but it is increasingly clear that national cooperative groups are uniquely well suited to perform practice-changing trials focused on comparative effectiveness of specific strategies and optimizing risk stratification for tailored approaches. In our new roles as co-chairs of the Alliance Breast Committee, we have had the opportunity to consider the landscape of breast cancer research, in which improvements in both local and systemic treatment, much of which came from Alliance or legacy group efforts, have both improved outcomes and reduced toxicity. Alliance and Alliance legacy groups have been at the forefront of improved therapy in the adjuvant and metastatic settings, and have been highly proactive in obtaining external funding to incorporate translational endpoints into trials that are redefining the next generation of trials. Especially in breast cancer, Alliance has led many patient-centered initiatives in patient-reported outcomes, documentation of disparities and efforts to reduce them, and health services research. Now it is time to determine our priorities for the next generation of clinical trials.
In Fall 2016, we surveyed the Alliance Breast Committee membership regarding key clinical questions and feasibility of recruiting to trials addressing those questions. Fifty-seven responding committee members including both academic and community-based investigators as well as patient advocates chose from among 11 broad research areas identified by the breast committee leadership. The findings of this survey were analyzed descriptively and refined in order to identify the top-ranking concepts (Table 1 below). The gaps in knowledge identified by this survey illustrate the changing culture of breast cancer research, in which the multidisciplinary team has evolved to include scientists, public health researchers, and policy mavens in addition to patient advocates and clinicians. Through the survey results, the Alliance Breast Committee communicated that the group should prioritize testing not only drugs and regimens but also incorporate biologic and heal th services advances. Excellent outcomes and long disease-free intervals for many breast cancer subsets complicate design of trials addressing some of the unmet clinical needs. Late recurrence in hormone receptor-positive (HR+) breast cancer, for example, is an example of such a challenge.
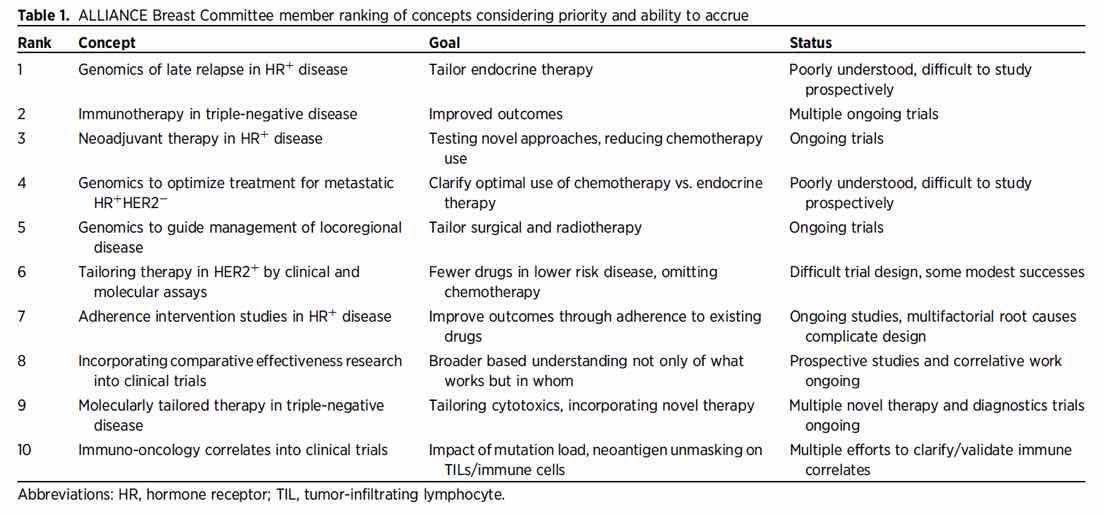
Recent trials have evaluated the role of extended adjuvant endocrine therapy for patients with early stage HR+ disease, often for more than 10 years after diagnosis. This strategy has been widely adopted. However, given the potential deleterious effects on bone and gynecologic health, clinicians have strong interest in means to more thoughtfully apply these therapies to those who need it the most. Illustrating this, the top-ranked p riority from the survey was refining risk stratification for late relapse using genomics in order to omit extended adjuvant endocrine thera py for those patients at low risk and to test novel endocrine therapy f or those at high r isk. While studies using RNA-based assays show promise in this realm, these have not been prospectively tested. The problem of considering such a trial is the low annual risk of relapse of 1-2% after the 5th year, making the required sample size and trial duration that would be required nearly insurmountable obstacles. An alternative strategy is to leverage large mature datasets with a high proportion of banked primary tissue to address biomarkers of late relapse. This may require collaboration and a “big data” approach incorporating genomics and biologic variables plus clinical data rather than a traditional prospective clinical trial. We believe that Alliance, along with other NCTN groups, should play a leading role in such initiatives given our well-annotated datasets including long-term follow-up from prior and ongoin g clinical trials, often with tumor specimens available for correlative testing.
The next-ranked clinical priority fell into the traditional cooperative group paradigm (incorporating immune checkpoint inhibitor therapy into adjuvant care) and there are a number of initiatives underway. Several other identified priorities were more translational in nature, including validating intermediate biomarkers for outcome using pre-operative therapy for ER-positive breast cancer, which is being addressed in the currently accruing Alliance study, ALTERNATE (NCT01953588). This paradigm-changing study is testing the clinical utility of an immunohistochemistry-based prognostic score, the PEPI score, and will examine other endocrine predictors as secondary objectives. Several clinical priorities center on moving away from relying primarily on ER and HER2 to determine treatment approaches; these included using RNA-based assays to guide choice of metastatic treatment in hormone receptor-positive disease, determining the need for dual HER2-targeting or less aggressive HER2-directed regimens, and tailoring radiation therapy to those at higher risk of locoregional failure. Integrating and targeting DNA aberrations into such clinical problem-solving requires additional study but is clearly an opportunity. The message from our survey is that the clinical investigator community has an appetite for clinical trials with a translational bent, even as the primary objective.
In addition to leveraging our inc reased understanding of the biologic underpinnings of breast cancer, several of the highest priority research gaps centered on disparities in outcome and improving delivery of care. Specific issues related to tackling the problem of treatment nonadherence and the need for rigorous determination of best therapies within and across diverse populations. These topics ranked highly in part because noninitiation/nonadherence is common, negatively impacts outcome, and particularly affects vulnerable breast cancer patients such as African-American women and those at both ends of the age spectrum. The survey also suggested that routine incorporation of comparative effectiveness analyses (the comparison of existing approaches to determine greatest benefits and harms) was appropriate and a priority for the cooperative group setting. We agree, especially given that comparative effectiveness trials are difficult to fund outside of the federal sphere and can have significant impact on public policy. In this era of health care reform and the need for rational though not nihilistic “less-is-more” strategies when possible, it will be as important to understand how well our treatment strategies work across populations and in various settings as it is to develop the strategy in the first place. Improved outcomes in breast cancer have not been universal, and illustrate the Fundamental Cause Theory of disparities, which holds that disparities in outcomes by race and resources are widest in the most treatable conditions. We must ensure that our underserved populations are not left behind, which requires that we invest in identifying barriers to access and care as a community; the cooperative group disease committees could play key roles in this kind of research. Among the initiatives we have begun is more defined relationships between the breast committee and committees working on topics relevant for breast cancer patients such as symptom intervention, cancer outcomes, and cancer disparities.
There is much work to be done and many challenges facing the Alliance Breast Committee and the broader cancer research community in addressing unmet clinical research needs in the future. We are excited about the level of investigator thoughtfulness and engagement in this future and that together, we in the Alliance Breast Committee will be able to design, conduct and learn from the next generation of innovative practice-changing trials.
For other articles in the Summer issue of the Alliance E-News newsletter, see below.
-
Spotlight on Alliance Trials
CALGB (Alliance) 10603 | FDA Approves New Combination Treatment for AML
Alliance 221208 | Managing Brain Radionecrosis (BeSt Trial)
Alliance A091202 | Intervention for Myxoid Liposarcoma
Alliance A091305 | Rare Tumor Subset for Advanced Anaplastic Thyroid Cancer - In the News
-
Protocols
Recent Alliance Protocol Activations - Alliance at ASCO 2017
-
Staff & Members
Meet New Alliance Board of Directors and Executive Committee
Meet New Alliance Leadership
Alliance Members on the Move -
Announcements
What Alliance Members Need to Know About CTEP's New Registration and Credentialing Repository (RCR) -
Featured Columns
Alliance Breast Committee Member Survey
Alliance Disparities Committee Corner - Did You Know? Alliance Website Tips
- Alliance Mission: By the Words


