E-Newsletter - May 2021
Message From the Group Chair
 MEETING CHALLENGES, BRINGING BENEFITS TO PATIENTS with CANCER
MEETING CHALLENGES, BRINGING BENEFITS TO PATIENTS with CANCER
Alliance Members and Colleagues,
During the past 18 months, when our world was disrupted by the COVID-19 pandemic, each of us met challenges that forced us to re-evaluate our personal and professional priorities. The priorities of our clinical trials cooperative group are to design studies that bring the greatest possible benefit to patients with cancer, and to make these trials available to everyone who needs them. Lately, we have been fighting on two fronts: treating cancer, the second leading cause of death in the U.S., and also COVID-19, which was third in line as a cause of mortality in 2020. Cancer did not stop during the pandemic, and neither did Alliance researchers. We made steady progress by “doing whatever it takes.”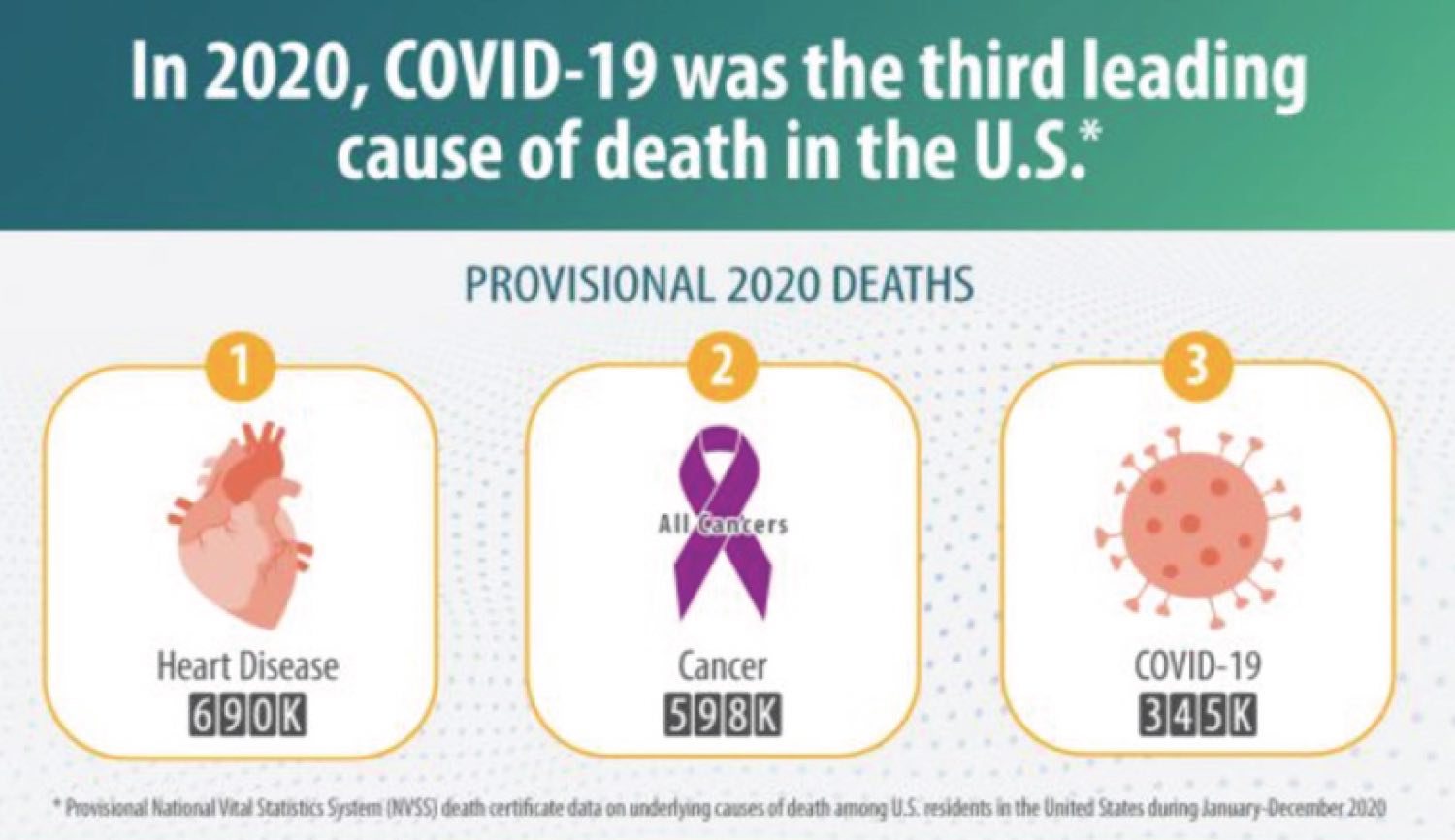
Even during routine times, “doing whatever it takes” means a lot of extra work on the part of Alliance members. Alliance scientific committees constantly challenge current practice by developing new trials, and this requires that each study development team, led by the study chair, statistician, and protocol coordinator, works for months and sometimes years, often without the assurance that the idea they are championing will make it to study activation. This dedication to moving forward despite uncertainty requires a truly remarkable level of commitment to the difficult process of clinical research. The results are inspiring, as shown by review of recent Alliance publications. For example, Alliance study C40601, for patients with HER2-positive breast cancer, showed that neoadjuvant chemotherapy with a combination of HER2-targeting drugs improved both relapse-free and overall survival, and also that gene expression signatures predicted pathologic complete response and recurrencefree survival, perhaps providing a means for rational escalation and de-escalation treatment strategies (Fernandez-Martinez, et al. J Clin Oncol 2020; 38:4184). A post hoc analysis of the Cabozantinib vs Sunitinib as Initial Targeted Therapy for Patients with Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk (CABOSUN) trial showed that in addition to producing superior progression-free survival, cabozantinib treatment provided longer quality-adjusted survival compared with sunitinib (Chen, et al. Cancer 2020;126:5311). In addition, a long-awaited report of the final overall survival results from the IDEA collaboration showed that for patients with stage III colon cancer, three months of post-operative adjuvant chemotherapy was not inferior to six months of treatment, with a substantial reduction in toxicities, inconveniences, and cost associated with the shorter treatment duration (Andre, et al. Lancet Oncol 2020; 21:1620). Each of these studies produced substantial improvements in clinical care. 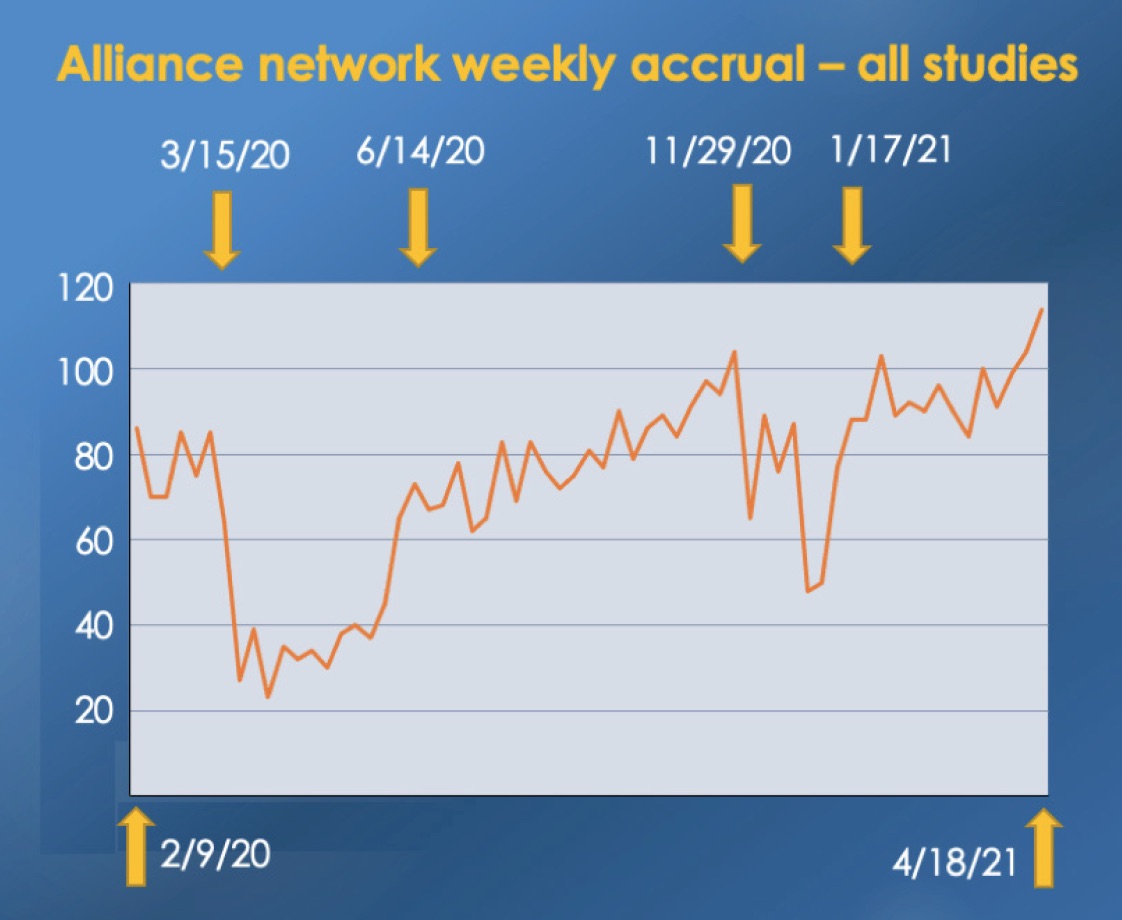
An equally dedicated army of Alliance clinicians, distributed across the entire U.S., contributes the considerable extra effort required to ensure that as many patients as possible have the chance to enroll on a clinical trial that can benefit them. Here again, Alliance researchers overcame the exceptional challenges imposed by the pandemic. An example of this is the Alliance accrual to NCI-sponsored trials over the past 18 months. Accrual fell below average during the winter 2020 and 2021 COVID-19 surges, and then rose dramatically as each surge waned, keeping total accrual almost equal to that of the pre-pandemic period. Alliance trials also reached more patients living in rural locations. Over the past two years, Dan Nikcevich and Tara Henderson have been leading the Rural Site Working Group, a team designing strategies to improve clinical trials access for patients living in rural locations. Their work has paid off. During 2020, the percentage of rural patients enrolled on Alliance trials increased by 22.6%.
During the 2021 Alliance Spring Virtual Group Meeting a few weeks ago, those who attended heard many more examples of progress by Alliance researchers. Congratulations and thanks to each of you. On behalf of all of our patients, I am profoundly grateful that Alliance research remains a priority for you, even during particularly challenging times.
Warm regards,

Monica M. Bertagnolli, MD
Group Chair
For other articles in this issue of the Alliance E-News newsletter, see below.
-
Message From the Group Chair - Monica M. Bertagnolli, MD
Message From the Group Statistician - Sumithra J. Mandrekar, PhD - Alliance Activates 11 New Trials Over the Past Year
- Alliance/AFT at 2021 ASCO
-
Alliance Scholar Awards
Daniel J. Sargent, PhD Memorial Fellowship in Innovative Clinical Trial Design and Methods Award - Alliance Spring Virtual Group Meeting Recap
-
Alliance in the News
New Operating Standard: mCODE Collaboration Is Bringing Uniformity to EHR Data
Trials Explore Whether Endocrine Therapy Can Delay or Avert Surgery in Low-Risk DCIS
Neoadjuvant Endocrine Therapy Use in Early Stage Breast Cancer During the COVID-19 Pandemic
New Data Support Preoperative Chemoradiation Regimen for Borderline Resectable Pancreatic Cancer
Electronic Geriatric Assessment: Is It Feasible in a Multi-Institutional Study That Included a Notable Proportion of Older African American Patients? (Alliance A171603)
How did a multi-institutional trial show feasibility of electronic data capture in older patients with cancer? Results From a Multi-Institutional Qualitative Study (Alliance A171902)
Randomized Phase III Trial of Gemcitabine and Cisplatin With Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance)


