FEATURED TRIAL: PROSPECT-LUNG TRIAL
First Study from NCI's Clinical Trials Innovation Unit

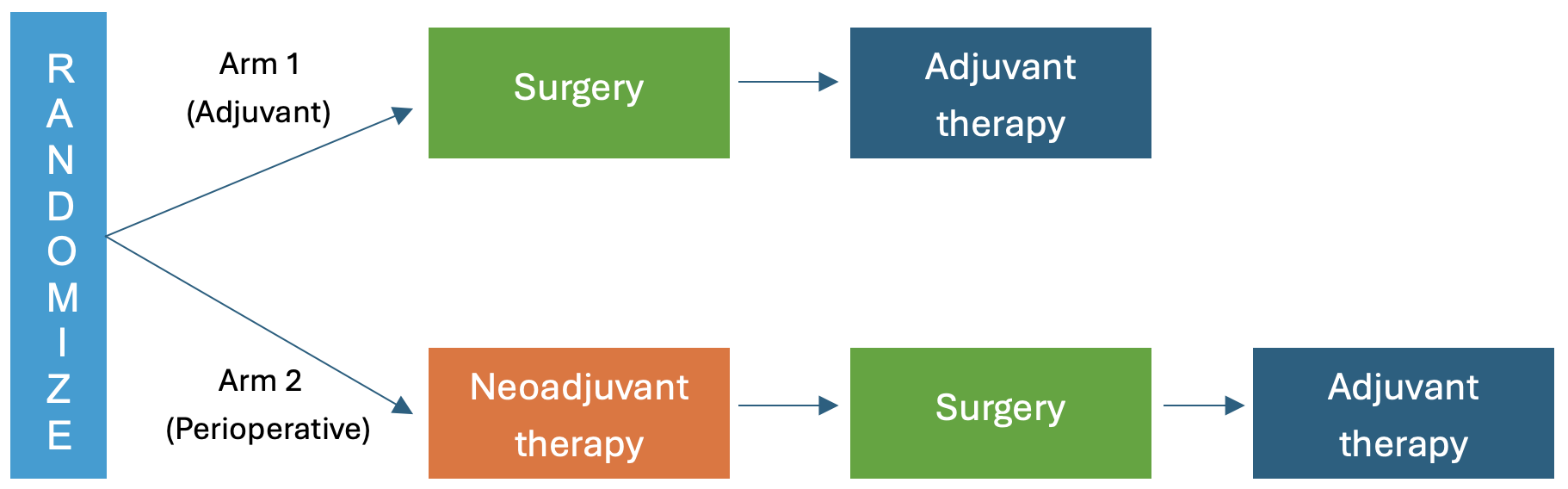
The PROSPECT-Lung trial is a large, multicenter trial developed and co-led by the Alliance for Clinical Trials in Oncology and the SWOG Cancer Research Network and conducted within the NIH-funded NCI National Clinical Trials Network (NCTN) with participation from the NCI Community Oncology Research Program (NCORP). The trial, which is the first to open through the newly formed National Cancer Institute (NCI) Clinical Trials Innovation Unit (CTIU), aims to evaluate the role of immunotherapy before and after surgery in patients with non-small cell lung cancer. It is designed to determine whether adding immune checkpoint inhibitors to standard chemotherapy, either before or after surgery, can help patients with resectable, early-stage non-small cell lung cancer live longer and stay cancer-free. The trial will also look at whether perioperative immunotherapy—given both before and after surgery—is more effective than just using it after surgery (adjuvant therapy).
The PROSPECT-Lung trial will enroll 1,100 patients across multiple sites in the United States with operable stage IIA to IIIB non-small cell lung cancer who have had no prior treatment for their lung cancer within the last 5 years. Patients will be randomized into two groups to receive perioperative therapy (treatment before and after surgery, also known as neoadjuvant/adjuvant therapy) or adjuvant therapy alone. Patients will receive standard of care treatment of their cancer based on the stage of the disease and in accordance with national guidelines. Patients will be followed for 10 years.
PRINCIPAL INVESTIGATORS

Daniel Morgensztern, MD
Alliance Study Co-Chair and Professor in the Division of Medical Oncology at Washington University, Siteman Cancer Center

Raid Aljumaily, MD
SWOG Study Co-Chair and Associate Professor in the Division of Hematology/Oncology at the University of Oklahoma, OU Health Stephenson Cancer Center

Raymond Osarogiagbon, MD
SWOG Co-Chair, Director of the Multidisciplinary Thoracic Oncology Research Program at Baptist Cancer Center

Linda Martin, MD, MPH
Alliance Co-Chair and Professor and Chief of Thoracic Surgery at University of Virginia, School of Medicine / UVA Comprehensive Cancer Center.
LEARN MORE ABOUT PROSPECT-LUNG
For more information about the PROSPECT-Lung trial (also known as CTIU2317-A82304-S2402), please contact the NCI’s Cancer Information Service at 1-800-4-CANCER or visit the NCI website at https://clinicaltrials.gov/study/NCT06632327.
- Alliance News Release
- SWOG News Release
- SWOG News: Pragmatica-Lung Closes, PROSPECT-Lung Opens
- OU Health - The University of Oklahoma Blog
- For NCTN Members: Visit the NCI Cancer Trials Support Unit (CTSU) website at http://www.ctsu.org and view the study activation webinar, patient flyer, patient information sheet, and PI/Site FAQs.
Official trial title: CTIU2317/A082304/S2402: Perioperative versus Adjuvant Systemic Therapy in Patients with Resectable Non-Small Cell Lung Cancer - PROSPECT Lung
For current accrual, visit CTSU website here.
The PROSPECT-Lung trial builds on promising data from earlier studies showing that adding immune checkpoint inhibitors to chemotherapy may improve survival in patients with lung cancer when used in the neoadjuvant, adjuvant, or perioperative settings. However, a critical question remains: Should immune checkpoint inhibitors be given before or after surgical resection? The trial is structured to answer this question.


